|
Product Information |
|
Product name |
Carfilzomib |
|
CAS No. |
868540-17-4 |
|
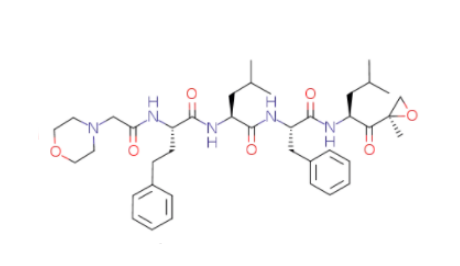
Molecular Formula |
C40H57N5O7 |
|
Molecular Weight |
719.922 |
|
Molecular Structure |
 |
|
Quality Standard |
98% up by HPLC |
|
Appearance |
White powder |
COA of Carfilzomib
|
ITEM |
SPECIFICATION |
RESULTS |
|
Appearance |
White powder |
Complies |
|
Identification |
IR, UF, HPLC. In comparison with Carfilzomib RS |
Complies |
|
Melting point(the Range of temperature≤4℃ ) |
230 ℃ |
230 ℃ |
|
Solubility |
slightly soluble in water and in alchohol, freely soluble in acetic acid and in methanol |
Complies |
|
Heavy metal |
0.001% - 0.002% |
Complies |
|
Loss on Drying |
≤1.0% |
0.23% |
|
Residue on ignition |
≤1.0% |
0.08% |
|
Residual organic solvents |
Ranged I and II toxic class |
Complies |
|
Total impurities |
≤1.0% |
0.54% |
|
Assay (by HPLC) |
≥ 98.0% |
98.96% |
|
Conclusion |
The results conforms with enterprise standard. |
|
|
Usage |
Studies have shown that lenalidomide combined with dexamethasone plus carfilzomib has unprecedented efficacy in the treatment of relapsed multiple myeloma. The three-drug combination therapy for patients with first relapsed multiple myeloma has a progression-free survival of more than two years, which confirms the efficacy of the three-drug combination. Although the interim data analysis of this trial did not yield median overall survival in either group, the addition of carfilzomib trended toward longer overall survival (73.3% in the added carfilzomib group and 65% in the control group). ), this test result did not exceed the statistical range set by the statistician at the beginning of the experiment. More importantly, the addition of carfilzomib did not significantly increase toxicity, and the quality of life score was also higher than that of the control group. Another important information brought by this trial is that the total effective rate of the three-drug combination is significantly higher than that of the two-drug combination, 87.4% and 66.9% in the two groups, respectively; the complete remission rate of the three-drug combination is more than 3 times higher, and the two Groups were 31.8% and 9.4%, respectively. By adding carfilzomib to the gold standard for the treatment of multiple myeloma, unprecedented duration of response was seen without increased toxicity, which holds great promise for relapsed and high-dose preconditioning patients. These results may establish a new standard of care for the treatment of relapsed and high-dose preconditioning patients.
*Products under the patent are only for R&D use