|
Product Information |
|
Product name |
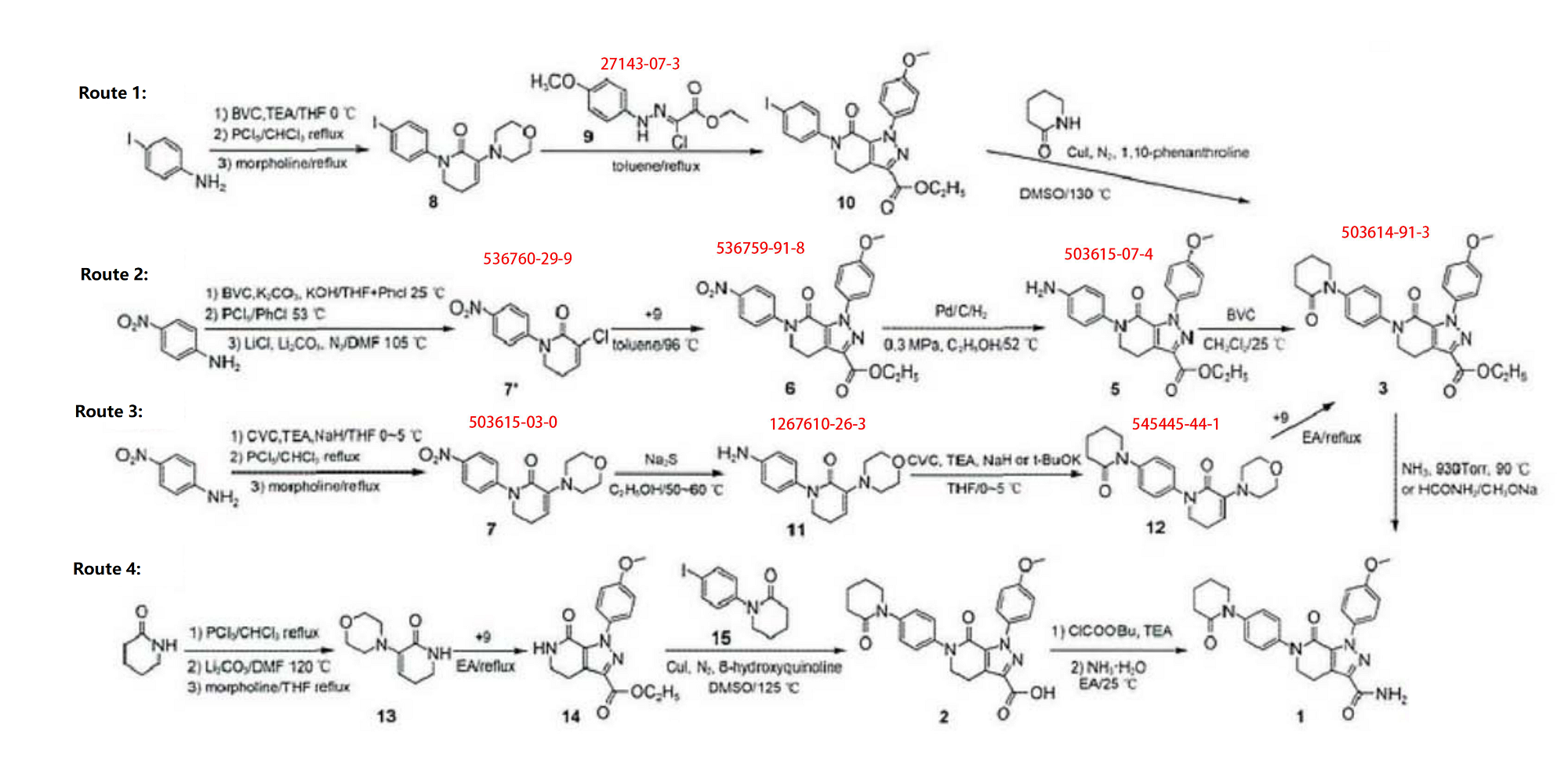
Apixaban intermediate; 5,6-Dihydro-3-(4-morpholinyl)-1-(4-nitrophenyl)-2(1H)-pyridinone; |
|
CAS No. |
503615-03-0 |
|
Molecular Formula |
C15H17N3O4 |
|
Molecular Weight |
303.318 |
|
Quality Standard |
99% up by HPLC; Any Other impurities<0.05% |
|
Appearance |
Almost white or white crystalline powder |
Synthesis Routes of Apixaban: