|
Product Information |
|
Product name |
Tenofovir Alafenamide Fumarate / TAF |
|
CAS No. |
1392275-56-7 |
|
Molecular Formula |
2(C21H29N6O5P).C4H4O4 |
|
Molecular Weight |
1069.00 |
|
Quality Standard |
98% up, Medicine Grade |
|
Appearance |
White powder |
|
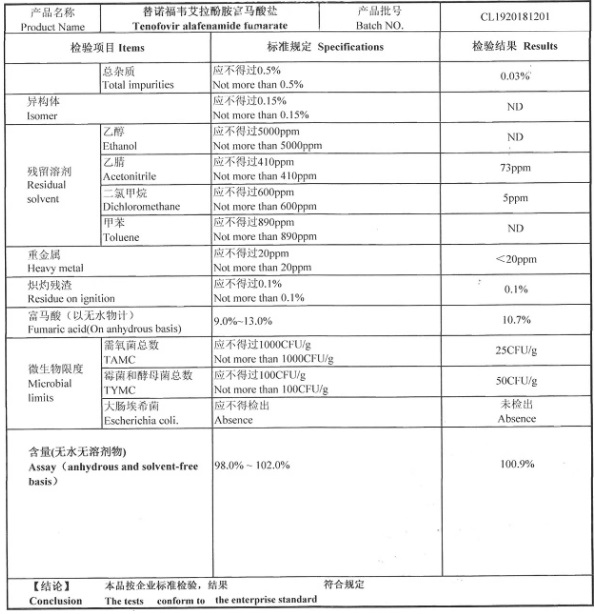
COA of Tenofovir Alafenamide Fumarate / TAF |
|
Usage |
Function of Tenofovir Alafenamide Fumarate / TAF
Tenofovir Alafenamide Fumarate / TAF is a novel nucleotide reverse transcriptase inhibitor for the treatment of chronic hepatitis B virus infection and compensatory liver disease.
Tenofovir alafluamine fumarate is an oral prodrug of tenofovir (TFV), a modified version of the commercially available drug tenofovir disoproxil fumarate (TDF). Compared with TDF, TAF has a smaller clinical dose and is easier to enter the cell. The virus acts more strongly and the adverse reactions are reduced.
API TAF was approved by the COS on November 10, 2016 for the treatment of adult patients with chronic hepatitis B infection. PMDA was approved on December 19, 2016 and approved by EMA in 2017.1.9, becoming the first hepatitis B drug approved for listing in Europe in the past 10 years. Approved for the treatment of HIV infection, all three drugs are based on tenofovir alafenamide fumarate.
*Products under the patent are only for R&D use