LIRAGLUTIDE 204656-20-2
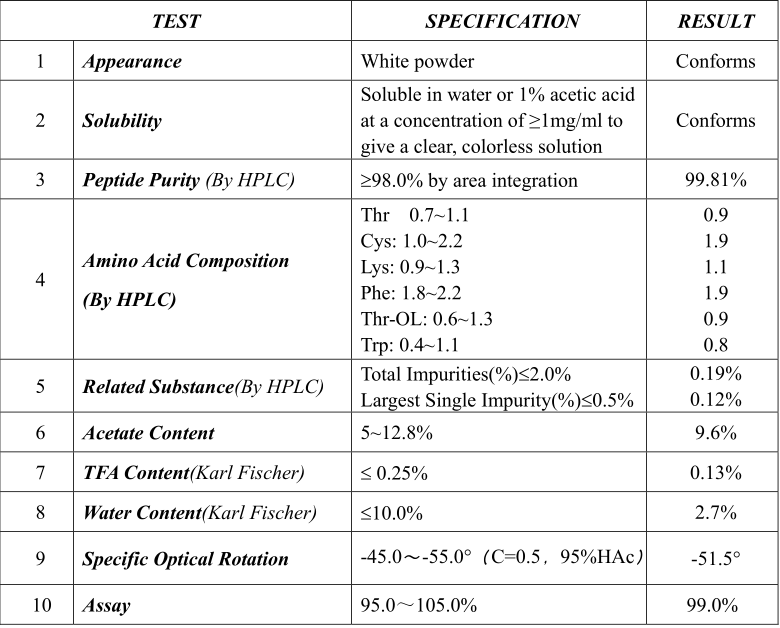
Product Information Product name Liraglutide Sequence H-His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu- Glu-Gly-Gln-Ala-Ala-Lys[N6-[N-(1-oxohexadecyl)-L-γ-glutamyl]- -Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH CAS No. 204656-20-2 Molecular Formula C172H265N43O51 Molecular Weight 3751.20 Storage 2~8℃ Appearance White to off-white crystalline powder COA of Liraglutide Test Specification Results Appearance: White to off-white crystalline powder or lumps White powder Identity: 3751.2±1.0 3751.1 Purity (By HPLC) : Not Less than 98.0%; 99.51% Residual solvents: ≤0.25%total; ≤0.1%individual; ≤0.01%CH2CN Complies Related Peptide Total Impurity ≤ 2.0% Largest Single Impurity ≤ 1.0% TI =0.41% LSI =0.25% Peptide Content: Not Less than 85.0% 87.7% Water (K.F.): Not more than 5% 4.2% Acetate acid: Not more than 10% 8.1% Bacterial Endotoxins Not more than 50IU/mg Complies Usage Function and Usage of Liraglutide 204656-20-2 1、 Type 2 diabetes Liraglutide improves control of blood glucose.It reduces meal-related hyperglycemia (for 24 hours after administration) by increasing insulin secretion (only) when required by increasing glucose levels, delaying gastric emptying, and suppressing prandial glucagon secretion. In common to various degrees with other GLP-1 receptor agonists, liraglutide has advantages over more traditional therapies for type 2 diabetes: · Liraglutide acts in a glucose-dependent manner, meaning it will stimulate insulin secretion only when blood glucose levels are higher than normal, preventing "overshoot". Consequently, it shows negligible risk of hypoglycemia. · Liraglutide has the potential for inhibiting apoptosis and stimulating regeneration of beta cells (seen in animal studies). · Liraglutide decreases appetite and inhibits body weight gain, as shown in a head-to-head study versus glimepiride. · Liraglutide lowers blood triglyceride levels. 2、Obesity Liraglutide has been approved as an injectable adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients. The specified criteria are an initial body mass index (BMI) of 30 kg/m2 or greater (obese), or 27 kg/m2 or greater (overweight), in the presence of at least one weight-related comorbid condition (e.g.hypertension, type 2 diabetes mellitus, or dyslipidemia). In late 2014, data were reported from the SCALE™ Obesity and Prediabetes trial, which is a randomised, double-blind, placebo-controlled, multinational trial in non-diabetic people with obesity and non-diabetic people who are overweight with comorbidities. In this phase 3a trial, there were 3,731 participants randomised to treatment with liraglutide 3 mg or placebo, both in combination with diet and exercise. Those who completed the 56-week trial achieved an average weight loss of 9.2%, to be compared with a 3.5% reduction in the placebo group.